
SynCardia total artificial heart is currently approved as a bridge for the transplant of patients suffering end-stage heart failure which affects both ventricles. It is the first and only total artificial heart approved worldwide by the FDA, Health Canada and EC (Europe).
Cardiomyopathy:
- Restrictive
- Infiltrative
- Hypertrophic
- Dilated
Congenital paediatric and adult conditions:
- Ventricular septal defect
- Transposition of the great arteries
- Failed Fontan circulation
Transplant rejection
Persistent ventricular tachycardia.
SynCardia total artificial heart is currently approved as a bridge for the transplant of patients suffering end-stage heart failure which affects both ventricles. It is the first and only total artificial heart approved worldwide by the FDA, Health Canada and EC (Europe).
Cardiomyopathy:
- Restrictive
- Infiltrative
- Hypertrophic
- Dilated
Congenital paediatric and adult conditions:
- Ventricular septal defect
- Transposition of the great arteries
- Failed Fontan circulation

Transplant rejection.
Persistent ventricular tachycardia.

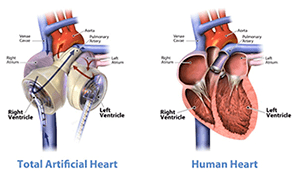
SynCardia Total Artificial Heart is made of two biocompatible plastic ventricles with four valves. Each ventricle has a diaphragm that separates the internal chamber (where blood flows) from the external chamber (where the pneumatic mechanism acts). The ventricles are connected to an external power supplied called “controller” by two small air tubes called “hoses”, providing a pulsatile flow. The hoses leave the abdominal wall of the patient just under the rib cage.
The unique design of the total artificial heart allows the patient’s body to adjust automatically blood flow according to the activity level.
During exercise, the increased muscular and body movements take more blood flow to the heart. There is no need to change heart rate as the body adjusts automatically the amount of blood pumped by the total artificial heart.
- 70 cc: Designed for patients with a body surface >1.70 m2
- 50cc: Designed for patients with a body surface between 1.2 and 1.85 m2
Proven reliability: During 30 years of use, the valves have never failed in SynCardia total artificial heart. The diaphragm, which is responsible for pumping blood in and outside the ventricles, has shown a failure index lower than 1% in over 1,000 implants. Currently the longest time that a patient has been assisted by the total artificial heart is 1,374 days (almost four years) before a successful heart transplant.
As with a heart transplant, SynCardia total artificial heart is the only device that:
Unlike a donor heart, SynCardia total artificial heart:
- Is available immediately at centres certified by SynCardia.
- Does not require expensive immunosuppressive agents, that can cause subsequent complications.
- Provides consistent quality and reliable performance.

SynCardia Total Artificial Heart is made of two biocompatible plastic ventricles with four valves. Each ventricle has a diaphragm that separates the internal chamber (where blood flows) from the external chamber (where the pneumatic mechanism acts). The ventricles are connected to an external power supplied called “controller” by two small air tubes called “hoses”, providing a pulsatile flow. The hoses leave the abdominal wall of the patient just under the rib cage.
The unique design of the total artificial heart allows the patient’s body to adjust automatically blood flow according to the activity level. During exercise, the increased muscular and body movements take more blood flow to the heart. There is no need to change heart rate as the body adjusts automatically the amount of blood pumped by the total artificial heart.

- 70cc: Designed for patients with a body surface >1.70 m2
- 50cc: Designed for patients with a body surface between 1.2 and 1.85 m2
Proven reliability: During 30 years of use, the valves have never failed in SynCardia total artificial heart. The diaphragm, which is responsible for pumping blood in and outside the ventricles, has shown a failure index lower than 1% in over 1,000 implants. Currently the longest time that a patient has been assisted by the total artificial heart is 1,374 days (almost four years) before a successful heart transplant.
As with a heart transplant, SynCardia total artificial heart is the only device that:
Unlike a donor heart, SynCardia total artificial heart:
- Is available immediately at centres certified by SynCardia.
- Does not require expensive immunosuppressive agents, that can cause subsequent complications.
- Provides consistent quality and reliable performance.
Implantation of SynCardia total artificial heart:
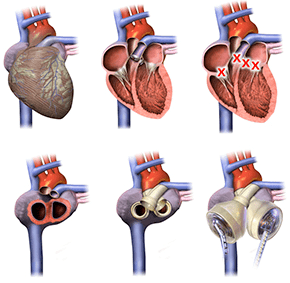
Due to its unique design, the total artificial heart does not require sensors, motors or electronic devices of any type inside the body.
All electronic devices are located safely outside the body in the external controller, which feeds the total artificial heart with air and vacuum pulses.
It is never needed to operate again for repairing or replacing electronic devices. SynCardia hospital controller impels the total artificial heart from implantation until the condition of the patient is stable.

Once stability is achieved, patients who meet the necessary requirements are then connected to the portable Freedom® controller, which allows the patient to move more easily at the hospital. Patients who meet the criteria for discharge can leave the hospital to wait for a compatible donor heart at home and in their communities.
Due to its unique design, the total artificial heart does not require sensors, motors or electronic devices of any type inside the body.
All electronic devices are located safely outside the body in the external controller, which feeds the total artificial heart with air and vacuum pulses.
It is never needed to operate again for repairing or replacing electronic devices. SynCardia hospital controller impels the total artificial heart from implantation until the condition of the patient is stable.

Once stability is achieved, patients who meet the necessary requirements are then connected to the portable Freedom® controller, which allows the patient to move more easily at the hospital. Patients who meet the criteria for discharge can leave the hospital to wait for a compatible donor heart at home and in their communities.
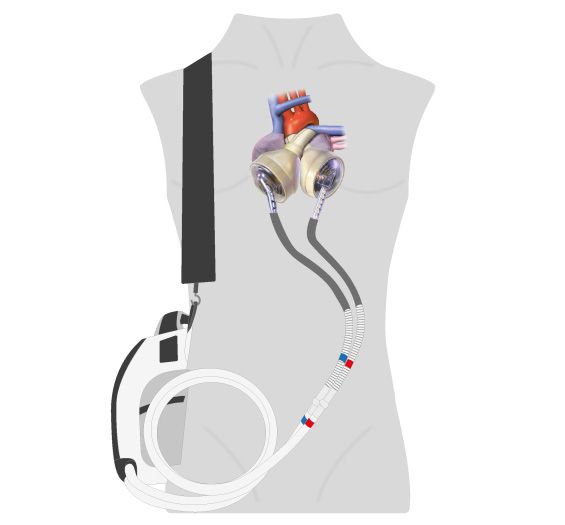
Freedom® portable controller is the first portable power source in the world for SynCardia total artificial heart.
With the use of Freedom controller, many patients can resume their normal activities. Freedom controller is powered by two lithium batteries that are recharged with a standard electrical output or the DC adapter of the car cigarette lighter.
This allows the patients to recharge their batteries while travelling and at night while sleeping. Patients can wear the Freedom controller in the Freedom backpack or shoulder bag.
Última actualización 20 March, 2023






